February 26, 2015
Wellesley, Mass., February 26, 2015 –BCC Research (www.bccresearch.com) reveals in its new report on laboratory-developed testing (LDTs), the market within the U.S. will grow from $9.7 billion in 2014 to about $17.7 billion in 2019, with a compound annual growth rate (CAGR) of 12.7%. In the realm of modern personalized medicine, key medical tests may serve important medical needs, but may not generate enough revenue to pay for the expenses incurred in the course of a regulatory filing. There are a number of analytes with which it is possible to get more precise and detailed measurements as compared to standard clinical laboratory testing. Laboratories offer these tests as a way of better serving patients.
Laboratory discoveries have long been a source of clinical diagnostic products and have routinely developed into FDA-regulated in vitro diagnostics. This transition involves a number of preclinical and clinical testing steps to validate both the accuracy of the test and the clinical relevance of the test prior to FDA marketing and approval. The goal is to make sure such a product is safe and adequate for the treatment and diagnosis of a disease, and the regulatory regime has been effective in protecting the public from clinical diagnostics that are not competent.
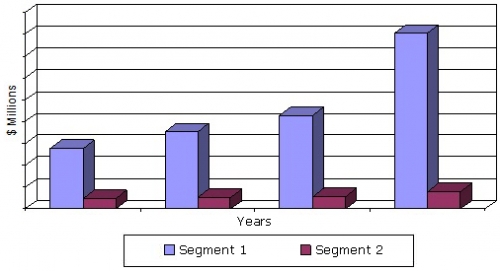
This market will be led by growth within the laboratory-developed clinical assay segment, which will increase from nearly $8.6 billion in 2014 to $16.1 billion in 2019, with a CAGR of 13.4%. The related analyte-specific reagent segment will grow from $1.1 billion in 2014 to about $1.6 billion in 2019, with a CAGR of 7%. This advancement will be paced by a variety of factors, such as the ramp up of genomic and molecular diagnostic assays.
While laboratories may offer tests for the same analyte, they differ on the techniques used and the information given, allowing for robust competition for service. “A large number of LDTs, particularly for common analytes tested using standard clinical diagnostic means, are covered using standard insurance for laboratory testing, while other tests require that patients pay out of pocket,” says BCC Research analyst Todd Graham. “The results of these tests are integrated into the spectrum of laboratory tests that health care providers use to diagnose and treat a wide number of health issues.”
Laboratory-Developed Testing: Technologies and Markets explores the unique regulatory scheme behind LDTs, how they are marketed and used, and the analyte-specific reagents necessary for development.
Editors and reporters who wish to speak with the analyst should contact Steven Cumming at steven.cumming@bccresearch.com.
Laboratory-Developed Testing: Technologies and Markets( HLC179A )
Publish Date: Dec 2014
Data and analysis extracted from this press release must be accompanied by a statement identifying BCC Research LLC as the source and publisher. For media inquiries, email press@bccresearch.com or visit www.bccresearch.com/media to request access to our library of market research.