Bioprinting of Human Microtissue for Drug Testing
Next-Gen Bioprinted Tissue Constructs for Drug Discovery

Drug development faces pressure to become faster, more predictive, and more human-relevant. The core problem is the apparent limitations in the way we test medication candidates before human trials. The primary issue with traditional testing methods, including 2D cell cultures and animal experiments, is their inability to replicate the dynamic biological processes that occur in human bodies. The lack of accurate representation in these models leads to unexpected clinical trial failures, resulting in massive financial losses and extended timeframes for drug approval.
Bio printed human microtissues aim to model human biology more closely than 2D cultures or animal studies, thereby supporting early detection and more informative screening. This study challenges the unmatched accuracy and predictability of 3D bio printed microtissues in drug screening. It emphasizes new developments in healthcare, recent technological advancements and ways that business executives can accelerate the uptake and further development of this ground-breaking invention.
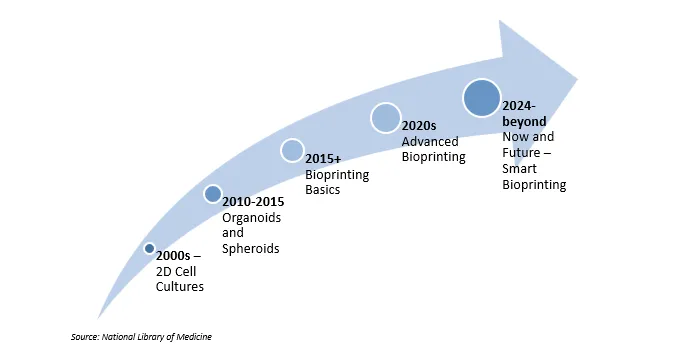
Evolution of Bioprinting for Drug Testing
Early bioprinting research in the 2010s focused on proof-of-concept tissues and simple hydrogel structures. Subsequent work enhanced cell viability, matrix composition and vascularization, allowing for the formation of thicker constructs and more physiologically relevant microenvironments. The latest platforms combine multi-cell printing with microfluidics and automated handling, making microtissues more compatible with high-throughput screening. As pilot studies accumulate in pharma R&D, the emphasis is shifting from feasibility to standardized workflows and validation.
The Traditional Drug Testing Bottleneck
The current drug-testing system depends on 2D cell cultures and animal laboratory models for its operations. The low cost of 2D cultures makes them suitable for high-throughput screening; however, their flat design fails to replicate cell-cell interactions, tissue-specific structures and extracellular matrix effects, resulting in incorrect assessments of drug efficacy and toxicity. The predictive value of animal models for drug testing outside their own species is limited due to differences between species.
Regardless of the current approaches, they mainly result in high attrition rates. Clinical attrition remains high from early trials to approval, increasing the need for better human-relevant models. To close this gap, the models need to mimic human tissue biology more closely. This would allow for better decision-making early in the process, avoiding even more expensive late-stage failures.
Why Bioprinted Human Microtissues Are Gaining Traction?
3D bioprinting fabricates living tissues by layering cells embedded in biocompatible "bioinks," precisely arranged using computer-aided design. This enables the creation of microtissues that recapitulate the physical, biochemical and mechanical properties of native human organs, including complex features, such as vascular networks and multiple cell types arranged in a spatially organized manner.
Recent Innovative Advancements Consist of:
- Volumetric and Rapid Bioprinting: Technologies like those created in the ENLIGHT project allow for the quick creation of viable tissues, such as a "mini-pancreas" with vascular-like channels. This enables realistic drug response testing and continuous nutrient supply. With the use of patient-derived stem cells, this model allows for customized treatment and enhances predictions of medication toxicity and efficacy.
- Vascularized Tissues: Perfusable microchannels that resemble blood vessels can be created to support thicker tissues for more extended periods of time and allow for testing in metabolically realistic environments that go far beyond basic cell aggregates or spheroids.
- Multicellular Complex Assemblies: It is possible to replicate crucial cell interactions that impact drug metabolism and responsiveness by printing cancer models, liver lobules, or cardiac tissues with different cell types organized precisely.
- Automated High-Throughput Systems: Some platforms, such as Inventia’s RASTRUM, deliver standardized 3D cultures in compatible formats for pharmaceutical screening pipelines, boosting efficiency and reproducibility.
- Nanoparticle-Enhanced Assembly: Emerging techniques use magnetic or light-sensitive nanoparticles to precisely organize cells, improving scalability and uniformity, crucial for screening large compound libraries.
Why does this Technology Matter Now More Than Ever?
Pharmaceutical companies face enormous pressures to deliver safer, faster and cost-efficient drug development. 3D bioprinting directly addresses these priorities by providing platforms that:
- Improve Predictability: More precise, human-like responses reduce clinical trial failure due to poor preclinical modeling.
- Improve Efficiency: Automated high-throughput bioprinting will reduce time-to-market development.
- Reduce Cost: Application of toxic or inefficacious candidates is lowered, coupled with a decrease in animal testing, which is both expensive and ethically questionable.
- Help Personalized Medicine: Patient-derived microtissues provide the capacity to profile drug response in an individualized manner, thereby enhancing therapeutic outcomes.
- Satisfy Regulatory Demands: Regulatory bodies, such as the FDA and EMA, are validating and recognizing the use of sophisticated models of human tissue.
Integrating 3D Bioprinting into Drug Development: The Healthcare Industry Perspective
Healthcare embraces digital and biofabrication innovations, with drug testing at the forefront. Biotech firms integrating bioprinted microtissues aim to streamline early screening and toxicity workflows with improved early-stage drug screening and toxicity assays. This technology also complements adjacent technologies, such as organ-on-chip and microfluidics, which provide dynamic tissue platforms that can simulate physiological forces on tissues (flow, stretch) closely resembling human organ function.
Recent healthcare trends have focused on precision medicine and the elimination of animal testing, both of which are driving the pharmaceutical industry's adoption of 3D bioprinting. Bioprinter models continue to garner growing acceptance and trust among clinicians and regulators, a vital aspect for eventual translation into the clinical setting and large-scale impact.
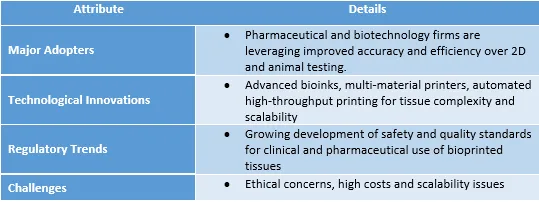
However, active collaborations among academia, industry and regulators, along with investments in automation and AI-guided optimization, are steadily addressing these barriers.
Action Steps for Industry Leaders
Consultancy firms and pharmaceutical stakeholders looking to future-proof drug development should:
- Evaluate and Pilot: Integrate bioprinted microtissues into early drug screening pipelines to validate improved predictivity and throughput.
- Partner Early: Collaborate with bioprinting tech firms and bioink suppliers to co-develop models tailored to therapeutic areas and compound classes.
- Engage Regulators: Work alongside regulatory bodies to shape guidelines and certification standards around bioprinted tissue assays.
- Invest in Cross-Discipline Expertise: Foster interdisciplinary teams merging cell biology, engineering, data science, and automation for successful adoption.
- Monitor Emerging Innovations: Stay abreast of advances like volumetric printing, nanoparticle assembly and integrated microfluidic systems.
Conclusion: The Imperative to Lead
Bioprinter human microtissues offer a practical approach to incorporating more human-relevant biology into preclinical testing. Near-term progress will stem from improved vascularization, automated workflows and more explicit validation criteria. Key hurdles, however, include standardization, cost and data comparability across laboratories. Over the next 12 to 24 months, expect focused pilots in toxicity and metabolism, with adoption driven by demonstrated gains in predictivity and operational fit.
Looking for Consulting & Advisory Projects
