Decentralized Clinical Trial Platforms Using Blockchain
Expert guidance to accelerate your market and strategy decisions.

Blockchain technology is rapidly being adopted in decentralized clinical trials (DCTs), bringing about a disruptive shift in the clinical research landscape. Conventional clinical studies are quickly becoming outdated because they are often costly, time-consuming and dispersed, and are susceptible to fraud or errors. Clinical trials can benefit from an unprecedented paradigm of data security, trust and efficiency—thanks to blockchain technology, which is an incorruptible, transparent, distributed ledger. To remain competitive and compliant in a rapidly evolving ecosystem, pharmaceutical companies, contract research organizations (CROs), regulators and technology suppliers must act immediately. This transition is not just a trend.
Blockchain-enabled decentralized clinical trials place patients at the center, promoting home-based study participation while cryptographically safeguarding data accuracy and transparency for investigators and regulators. The convergence of blockchain with smart contracts, AI and IoT catalyzes a new era where trial data integrity is unquestionable, consent is dynamic and transparent, and operational efficiency soars.
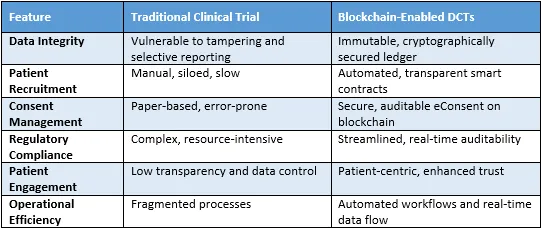
Blockchain DCT Versus Traditional Trial Comparison
One of the main impeding factors for clinical trials is the massive and sensitive datasets they rely on, which have been gathered from diverse sources, including wearables, electronic health records (EHRs), laboratories, imaging centers and pharmacies. These data are siloed and fragile to errors, fraud and delays in traditional systems. The decentralized structure of blockchain technology disbands such silos, thus providing an environment that can interoperate and maintain an incorruptible record trail, accessible only to authorized stakeholders.
Emerging Trends and Innovation Highlights
- Decentralized Recruitment and eConsent: Blockchain platforms enable faster recruitment while maintaining patient autonomy by using smart contracts for open eConsent processes, automated patient matching and real-time eligibility monitoring.
- Data Tokenization and Exchange: Data from clinical trials is converted into secure, tokenized digital assets, thus allowing simple data sharing with authorized parties and facilitating collaboration among global research networks.
- Integration with IoT and AI: IoT devices securely collect real-time patient data and transmit it, while AI algorithms rely on the reliable blockchain data to detect errors, forecast clinical trial results and support protocol adherence.
- Multi-Stakeholder Consortia: Pharmaceutical companies, CROs, healthcare providers and regulators are joining forces to develop interoperable blockchain standards that facilitate the coordination and scaling of DCTs through globalization.
Recent Development
- In May 2025, ABM Respiratory Care and Delve Health joined forces to initiate a multi-center home care research study. The effectiveness of ABM's BiWaze Clear System for patients with bronchiectasis will be assessed in this decentralized clinical research. In particular, the study will monitor the system's impact on respiratory health in a home-based setting over six months.
- In August 2023, Parexel and Partex formed a strategic partnership to leverage Artificial Intelligence (AI)-driven solutions, aiming to accelerate drug discovery and development processes for biopharmaceutical clients worldwide. The partnership also sought to minimize risks associated with their respective asset portfolios.
- In August 2023, Novo Nordisk announced its acquisition of Inversago Pharma as part of its strategic initiative to develop new therapies for individuals with obesity, diabetes and other major metabolic diseases.
This transition represents not only operational improvement but also an evolutionary leap in clinical research methodology. Decentralized blockchain DCT platforms promise faster, more inclusive and higher-quality evidence generation, aligning perfectly with contemporary demands for personalized medicine and adaptive trial designs.
Challenges and Call to Action
- Scalability: Most existing public blockchains suffer from throughput limitations; thus, they require the use of an innovative solution or a hybrid model that combines on-chain and off-chain data management to overcome these constraints.
- Regulatory Harmonization: The attitude of regulatory agencies worldwide is one of caution as they are waiting for a clear set of standards and guidelines that will guarantee that blockchain systems are following privacy, ethical and legal norms.
- Data Privacy and Key Management: The act of ensuring stringent data privacy, even in transparent blockchains, and at the same time making sure that the private keys are securely managed for all the users, is still a highly complex process.
- Technology Integration and Cost: The trouble of many organizations in achieving full integration with their clinical trials management systems and the obtaining of an initial investment are just some of their practical obstacles.
The industry stakeholders who decide to adopt the DCT platform, powered by blockchain, will be the first to benefit and will be seen as early innovators. If they fail to do this, they will be left behind as this technology is turning from a pilot of promise into an industry standard.
Future Outlook
The decentralized clinical trial space, powered by blockchain technology, is a seismic change that will redefine clinical research for the next several decades. The blockchain in clinical trials market is predicted to grow at a rapid rate by 2025, when it will account for nearly half of the healthcare blockchain activity. The combination of blockchain, AI, IoT, and cloud technology creates an ideal environment for the triumvirate of transparency, compliance, and efficiency. Among the issues faced by blockchain networks are their scalability, regulatory compliance across different nations, the privacy of the data even when the ledger is transparent, and the ability of old systems to work with new ones. Nevertheless, technological advancements and the policy instruments already in place will accelerate in addressing these challenges. Industry leaders must seize the opportunity before them to implement blockchain-enabled DCT platforms or risk being left behind. Early adoption of technologies unlocks a wide rang
Looking for Consulting & Advisory Projects
